Browse Topics
- UK Responsible Person Services
- Who is a UK Responsible Person?
- Why Choose Taevas for UK Responsible Person Services?
- Taevas growth snapshot
- Industries We Serve
- Taevas services
- Download brochure
- Featured webinar
- Our global presence
- What customers say about us?
- Our case studies
- FAQs
- Taevas Linkedin profile
UK Responsible Person Services
Struggling with UK regulatory compliance? Taevas offers comprehensive UK Responsible Person services to help clients navigate regional complexities while entering the UK market.
The UK, being a superpower in the life sciences sector, demands stringent regulatory adherence across all phases of market entry, from product development to distribution. These regulations are designed to ensure that only safe, high-quality products reach consumers, making compliance a top priority for manufacturers.

Who is a UK Responsible Person?
A United Kingdom Responsible Person (UKRP) is an entity based in the UK that represents a manufacturer located outside the UK, fulfilling specific responsibilities to ensure the manufacturer complies with regulatory obligations.
Why Choose Taevas for UK Responsible Person Services?
At Taevas, we understand the complexity of UK regulations and provide the expertise needed to navigate them effectively. With our UK Responsible Person services, you can confidently meet the regulatory standards, avoiding costly delays or compliance issues while ensuring your products are successfully launched and maintained in this critical market.


- Act as your UK Responsible Person before health authorities, ensuring full compliance for product approvals.
- Serve as the liaison between your team and health authorities, handling all communications.
- Coordinate and attend health authority meetings as required.
- Submit product applications/dossiers for seamless market entry.
- Manage health authority fee payments with precision.
- Provide consistent follow-ups on submissions to keep projects on track.
- Address queries and secure approvals with expertise.
- Handle product lifecycle management, including variations and renewals.
- Trusted by 258+ global clients for regulatory compliance and market entry success.
- With extensive knowledge across 23 markets, we ensure seamless compliance with international regulations.
- Our strong relationship with global regulatory authorities expedites the approval process, facilitating quicker market access.
- We mitigate delays in market entry that can lead to financial losses.
- We minimize the risk of non-compliance, preventing costly recalls and penalties.
- We offer end-to-end support that encompasses all aspects of regulatory compliance, from product registration to market entry, distribution, warehouse & inventory management in global markets.
- We deliver customized strategies that address the unique regulatory needs of each market, ensuring optimal outcomes.
- Act as your UK Responsible Person before health authorities, ensuring full compliance for product approvals.
- Serve as the liaison between your team and health authorities, handling all communications.
- Coordinate and attend health authority meetings as required.
- Submit product applications/dossiers for seamless market entry.
- Manage health authority fee payments with precision.
- Provide consistent follow-ups on submissions to keep projects on track.
- Address queries and secure approvals with expertise.
- Handle product lifecycle management, including variations and renewals.
- Trusted by 258+ global clients for regulatory compliance and market entry success.
- With extensive knowledge across 23 markets, we ensure seamless compliance with international regulations.
- Our strong relationship with global regulatory authorities expedites the approval process, facilitating quicker market access.
- We mitigate delays in market entry that can lead to financial losses.
- We minimize the risk of non-compliance, preventing costly recalls and penalties.
- We offer end-to-end support that encompasses all aspects of regulatory compliance, from product registration to market entry, distribution, warehouse & inventory management in global markets.
- We deliver customized strategies that address the unique regulatory needs of each market, ensuring optimal outcomes.
Taevas Growth Snapshot
Brands Launched Globally
Growing Markets
Digital Revenue Across Industries
Industries Globally
UK Responsible Person for Medical Devices
A UK responsible person for medical devices is defined as a person established in the United Kingdom who acts on behalf of a manufacturer established outside the United Kingdom in relation to specified tasks with regard to the manufacturer’s obligations under the regulations’ to get the UK Conformity Assessed (UKCA) mark, a logo that indicates that the medical device is safe and meets the UK’s regulatory requirements for sale in Great Britain. The UKCA mark replaced the CE mark after the UK left the European Union.
All medical devices, including in vitro diagnostic medical devices (IVDs), custom-made devices and systems or procedure packs, must be registered with the Medicines and Healthcare products Regulatory Agency (MHRA) before they can be placed on the Great Britain (England, Scotland, and Wales) market.
Roles & Responsibilities:

UK Responsible Person for Cosmetics
All cosmetic products available to consumers must have a ‘Responsible Person’ who makes sure safety measures are followed and legal obligations are met.
A Responsible Person can be a business or an individual (including sole traders). A Responsible Person must have a UK established address to make cosmetic products available in GB. A UK established address does not include mail forwarding or PO box addresses.
A UK Responsible Person for cosmetics can be either:
- the manufacturer
- the importer
- the distributor, if they label the product as their own (for example, using their brand name)
- an appointed company or person (who is named by the manufacturer or the importer)
Roles & Responsibilities:

UK Responsible Person for Food & Dietary Supplements
The Food Standards Agency (FSA) oversees food safety and hygiene across the UK. Since Brexit, the UK has established new regulatory requirements for food and dietary supplements. Now, every food product must have a clearly identified Food Business Operator (FBO) based in the UK, whose address must appear on product labels.
Key Regulatory Requirements:
- While registration for imported foods and dietary supplements is generally not required, certain regulated foods, such as novel foods, require pre-market safety assessments and authorization.
- Dossier compilation and consultations with the relevant Health Authority (HA) are necessary for regulated food products.
- Novel food registrations require pre-market safety evaluations and approval before being marketed in the UK.
At Taevas, we assist manufacturers in navigating these regulatory complexities, offering end-to-end support to streamline market entry activities.
UK Responsible Person for Pharmaceuticals
To introduce medicinal products in the UK, the manufacturer should adhere to the stringent Regulatory issued by the Medicines and Healthcare products Regulatory Agency (MHRA). Taevas provides UK Responsible Person (UKRP) services to pharmaceutical companies and assists them in strictly fulfilling all the compliance requirements necessary for safe navigation within the complexities of the UK.
Roles & Responsibilities:

Regulatory Compliance: Ensures all technical documentation and clinical trial approvals are completed according to MHRA guidelines.
Liaison with MHRA: Act as the point of contact between the manufacturer and the MHRA, managing submissions, communications, and compliance.
Quality Control: Oversee adherence to Good Manufacturing Practices (GMP), ensuring safe and compliant product batches are released into the UK market.
Labeling & Packaging Compliance: Ensure product labeling and packaging meet UK-specific requirements, including safety information and regulatory standards.
Risk Management: Proactively identify and mitigate risks to prevent delays or recalls, ensuring continued product safety and compliance.

Discover the Full Spectrum of Our Services
Get expert insights via our recorded webinar

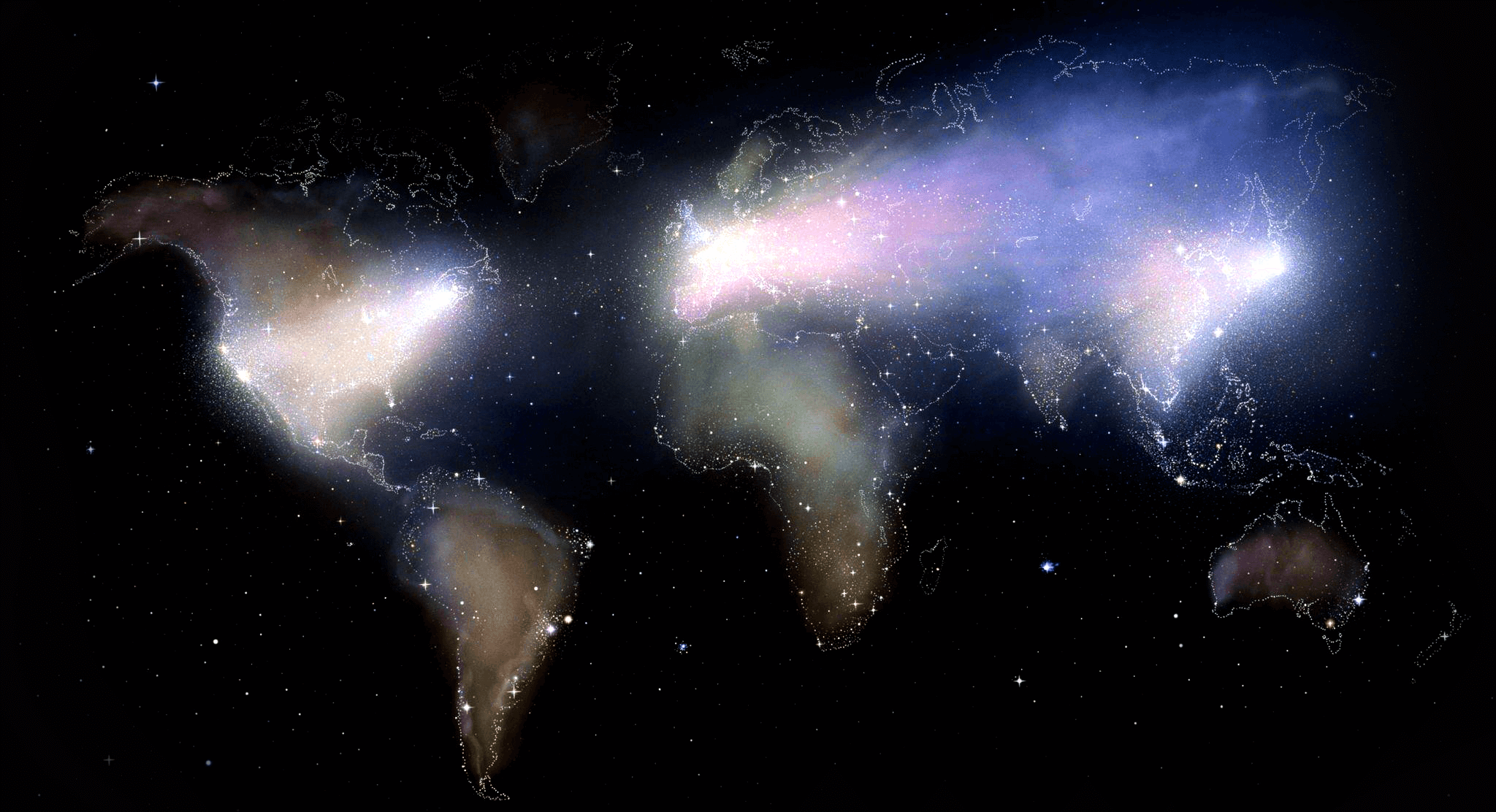
Our global presence
Taevas helps global life sciences innovators with global expansion strategies to connect with audiences worldwide and make it accessible for millions of people around the world.
Our global presence
Taevas helps global life sciences innovators with global expansion strategies to connect with audiences worldwide and make it accessible for millions of people around the world.

JAPAN

BRAZIL

UK

CANADA

MEXICO

SINGAPORE

MALAYSIA

SOUTH AFRICA

SRI LANKA

AUSTRALIA

POLAND

FRANCE

SWITZERLAND

China

Philippines

New Zealand

Vietnam

Taiwan
Chile

Peru

South Korea

Colombia
France

Nigeria
Thailand

Colombia
Our Clients Review






Frequently Asked Questions
What is a UK Responsible Person (UKRP)?
A UKRP is an individual or entity in the UK that ensures compliance with UK regulations for manufacturers outside the UK.
Why do medical devices need a UKRP?
A UKRP ensures regulatory compliance, such as obtaining the UKCA mark and registering devices with the MHRA.
What does a UKRP do for medical devices?
They handle documentation, collaborate with the MHRA, monitor complaints, and ensure post-market safety.
Can a non-UK entity be a Responsible Person for cosmetics?
No, the Responsible Person must have a UK-based address.
Who can act as a Responsible Person for cosmetics?
A manufacturer, importer, distributor (rebranding the product), or an appointed UK-based entity.
What does a UKRP do for food and dietary supplements?
They ensure compliance with food safety standards and manage regulatory approvals for novel foods.
Why do pharmaceutical manufacturers need a UKRP?
To ensure compliance with MHRA regulations and manage product approvals and quality control.
Taevas LinkedIn Profile
Schedule a free consultation With Taevas